General Research Interests
If the sex steroid hormones testosterone and estrogen are powerful enough to alter development, reproductive behavior and sex drive, how do they affect the body the rest of the time?
Members of the Viau lab explore sex differences and roles for the sex steroid hormones to influence normal stress coping responses and where this occurs within the central nervous system. Understanding circuit, transmitter and cellular mechanisms of stress in the rodent brain provides insight into why some disorders linked to stress are seen more commonly in one sex than the other, including mood, metabolic and cardiovascular disorders.
The hypothalamic-pituitary-adrenal (HPA) axis is an important hormonal system in humans and rodents, which ultimately controls the secretion of glucocorticoids from the adrenal gland: cortisol in humans, and corticosterone in rats.
Stress-induced activation of the HPA axis resulting in acute elevations in circulating glucocorticoids levels protect the organism from physiological insult by regulating a variety of physiological processes. For example, they provide adequate substrate for increased metabolic need and help to sustain blood pressure and depress immune function. On the other hand, chronic elevations in glucocorticoids produced by repeated stress exposure have been implicated in the pathogenesis of several forms of systemic, neurodegenerative, and affective disorders.
Sex steroid hormone receptors are not only distributed within brain regions affecting reproductive behavior and sex drive, but also within those mediating hormonal, behavioral and autonomic responses to homeostatic threat (i.e. stress). As estrogen and testosterone can alter the magnitude of anyone of these stress response systems, understanding where and how this occurs in the brain helps explain why some individuals thrive in the face of life stressors, while others are predisposed to disease.
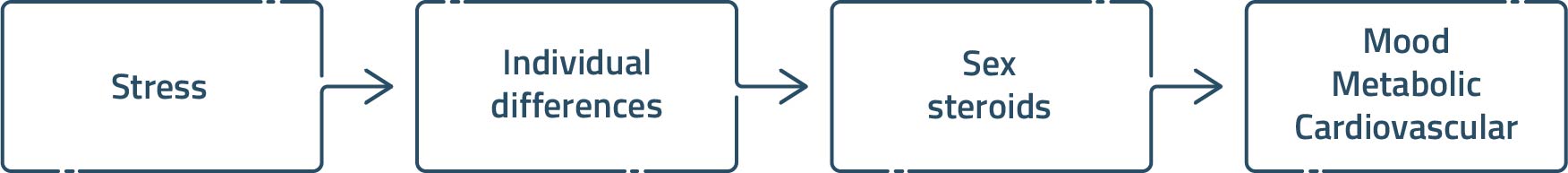
Diagram illustrating our overarching hypothesis that the sex steroid hormones (e.g. androgens and estrogens) provide a basis for understanding individual differences in stress reactivity under normal conditions, as well as those that produce pathological changes in mood, metabolic and cardiovascular tone. Note that sex steroid hormone secretion and signaling are also themselves subject to homeostatic threat, and in this design, can be placed either above or below “individual differences”; implying that relationships between gonadal status and stress can change in a situation- and context-dependent manner.